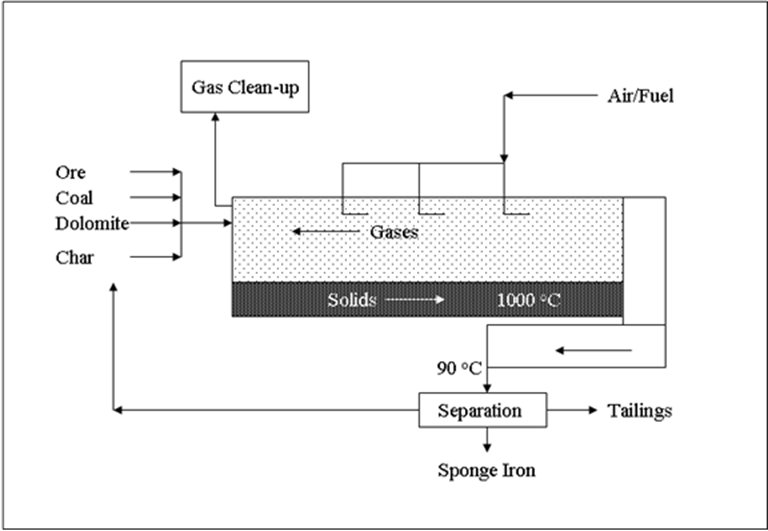
- Direct Reduction Iron (DRI) via the Stelco, Lurgi and Republic National (SL/RN) process.
A 2-step process beginning with the Boudouard reaction whereby the carbon in the char, a product of the coal devolatilization reaction in the preheat zone, reacts with CO2 to yield CO. The CO in, turn, reacts with the iron sand or iron oxide pellet to metallic iron i.e.,
C + CO2 = 2CO
Fe2O3 + CO = 2Fe + 3CO2 or, FeO + CO = Fe + CO2
yielding an overall reaction,
Fe2O3 + 3C = 2Fe + 3CO or, FeO + C = Fe + CO
- The success of the carbonaceous reduction of iron oxide opened doors for the beneficiation of other minerals which coexist with oxides of iron also known as mineral sands such as titanium dioxide (ilmenite ore) and nickel oxide (laterite ore). The Becher Process for the carbothermic reduction of ilmenite involves the roasting of titaniferous materials TiO2.
- Prior to this beneficiation of (Fe2O3. TiO2) is carried out via the carbothermic reactions to upgrade ilmenite by selective removal of the iron and thereby enrich the ore to TiO2 or what is industrially known as synthetic rutile.
