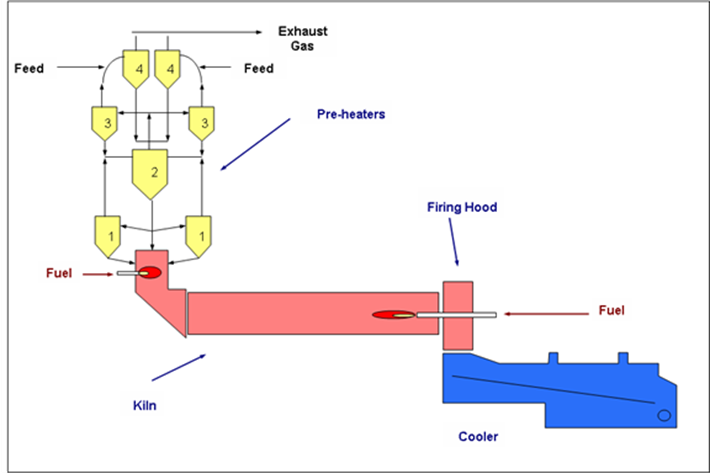
Feed – The raw mix or initial reactants of cement kiln charge comprises some formulations of limestone (CaCO3), alumina (Al2O3), hematite (Fe2O3) and silica (SiO3).
Thermal process transformation – the journey through the kiln exposes feed to drying, preheating, chemical reactions, a phase change, restructuring or sintering and cooling.
High-temperature Reactions – At the high temperature zones quicklime, alumina, ferric oxide, silica and other metal oxides react to form four main compounds of cement i.e., CaO.SiO2 (C3S), 2CaO.SiO2 (C2S), 3CaO.Al2O3 (C3A), and 4CaO.Al2O3.Fe2O3 (C4AF). Three high-temperature zones can be delineated:
(i). Decomposition Zone (900°C): small amounts of CaO.Al2O3 (CA), CaO.Fe2O3 (CF), 2CaO.Fe2O3, and 5CaO.3Al2O3 (C5A3) are formed.
CaCO3 = CaO + CO2
CaO + Al2O3 = CaO•Al2O3
CaO + CaO•Fe2O3 = 2 CaO•Fe2O3
3(CaO•Al2O3 + 2 CaO = 5 CaO •3Al2O3
(600-900°C)
(800°C)
(800°C)
(900-950°C)
After the decomposition zone, the axial temperature is greater than 900°C and one can assume that the dissociation reaction of the calcium carbonate (Equation 10.6), an endothermic reaction ΔH = -1660 kJ/kg CaCO3, is essentially complete.
(ii). Transition zone (900 – 1300 oC): The key reactions in this zone are exothermic beginning with silica (C2S), an exothermic reaction (DH = +603 kJ/kg C2S) followed by C4AF (DH = +109 kJ/kg C4AF), and C3A (DH = +37 kJ/kg C3A), i.e.,
2CaO + SiO2 = 2CaO•SiO2
3(2CaO•Fe2O3)+5CaO•3Al2O3+CaO = 3(4CaO•Al2O3•Fe2O3)
5CaO•3Al2O3+4CaO = 3(3CaO•Al2O3)
(1000°C)
(1200-1300°C)
(1200-1300°C)
(iii). Sintering Zone (1300 – 1400 oC): In this zone liquidus phase reactions are formed and the main component C3S (DH = +448 kJ/kg C3S) is formed by reaction between C2S and free lime as,
CaO•SiO2+ CaO = 3CaO•SiO2
(1350-1450°C)
Cooling to ambient temperatures via various coolers.
